The role of passive leg raise during cardiopulmonary resuscitation in sudden cardiac arrest: a systematic review and meta-analysis
Article information
Abstract
Objective
Passive leg raise (PLR) is an experimental cardiopulmonary resuscitation (CPR) technique involving elevation of the lower limbs from the horizontal plane during CPR. This procedure is thought to improve clinical outcomes in cardiac arrest. By comparing the use of PLR to conventional CPR, we aimed to evaluate survival outcomes and secondary outcomes of neurologically intact survival and return of spontaneous circulation (ROSC).
Methods
In this systematic review, databases of PubMed, Embase, and Cochrane Library were searched for relevant studies from inception to May 1st, 2021. Studies were included if they reported the use of PLR during CPR. Random-effects meta-analysis was performed for the outcome of survival to 30 days.
Results
The search yielded 554 articles, of which six met the criteria for inclusion (three animal and three human studies). The most common implementation of PLR was elevation of the heels to a 20- to 45-degree angle at the hips. Two human studies reported higher survival at hospital admission for patients that underwent PLR, though the difference was not significant. Two human studies reported lower ROSC and neurologically intact survival, while two animal studies reported higher ROSC and neurologically intact survival. The pooled effect on survival to 30 days/survival to hospital discharge was not significant (P=0.68), although our analysis showed a trend favoring PLR-CPR.
Conclusion
Despite several animal studies showing benefit from PLR-CPR, there are no human data supporting its use in human cardiac arrest. Future research needs to ascertain the best positioning during CPR to optimize clinical outcomes.
INTRODUCTION
Sudden cardiac arrest (SCA) is a life-threatening medical condition highly-relevant to both out-of-hospital and inpatient settings. It involves the sudden cessation of cardiac activity where the victim becomes unresponsive with abnormal breathing and absence of circulation [1]. If active interventions are not started rapidly, SCA progresses to sudden cardiac death (SCD) [1]. Each year, SCD accounts for almost 25% of 17 million deaths worldwide, making it one of the most common causes of death [2]. Early commencement of effective cardiopulmonary resuscitation (CPR) improves survival [3–5]. Yet, clinical outcomes have remained poor, with a pooled global 30-day survival rate for out-of-hospital cardiac arrest (OHCA) patients of 10.7% [6]. Hence there remains an urgent need to discover therapeutics to improve clinical outcomes.
Passive leg raise (PLR) is an experimental CPR technique which involves elevation of the lower limbs from the horizontal plane during CPR [7]. It is a simple maneuver that works through intravenous volume expansion—where blood is rapidly shifted from the lower extremities towards the intrathoracic compartment to improve venous return [8]. Prior to 1992, it was recommended as part of the International Standards and Guidelines for CPR [9], but was subsequently removed due to paucity of supporting clinical evidence. Recent years have seen a resurgence of interest in this topic due to its ease of applicability and plausible physiological basis for improving cardiac output during CPR [8]. Various animal and human studies have suggested better neurological outcomes [7], as well as an improved cardiac preload and blood flow during chest compressions [10].
With renewed interest in the role of PLR in the treatment of SCA [11], there is a need to consolidate the literature, both preclinical and clinical, to clarify the role of PLR in SCA. In this systematic review and meta-analysis, we primarily hypothesized that PLR improves survival (30-day survival or survival to discharge) in SCA as compared to conventional (supine) CPR (C-CPR). We further hypothesized that PLR improves secondary outcomes, namely, neurologically intact survival and return of spontaneous circulation (ROSC). Clarity on the effectiveness of PLR in SCA will inform CPR techniques, translating to improved clinical outcomes.
METHODS
Literature search strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12], and has been submitted to PROSPERO (ID: 281527). A comprehensive literature search was performed on PubMed, Embase and the Cochrane Library from inception to 1st May 2021. The search strategy was developed in consultation with a medical information specialist. The search strategy consisted of different combinations of the following search terms: leg declin*, PLR, leg elevat*, leg inclin*, leg ramp, leg rais*, passive leg rais*, resuscitation, CPR, cardiac arrest, CPR, cardiopulmonary resuscitation, and chest compress*. The search was performed by 2 independent reviewers (YKT & AFWH). During study screening for relevance, any disagreement was resolved by consensus with a senior author (MEHO). The reference lists of these articles were also screened, and hand-searched to identify further relevant studies.
Study and cohort selection
All studies (case reports, case series, preclinical studies, clinical trials and observational studies) that reported the use of PLR during CPR were included during the initial search. We subsequently excluded all studies that reported on other positions during CPR (such as head-up CPR), studies that did not have primary data, and those without an English translation.
Data extraction
Relevant quantitative data were extracted by 2 authors (YKT & AFWH) in the form of absolute counts and frequencies of events when appropriate. Any disagreement was resolved by consensus with a senior author (MEHO). Where available, the data included ROSC, survival to 30 days, survival to hospital discharge, neurologically intact survival, neurological outcomes, cerebral perfusion pressure, intracranial pressure, brain blood flow, coronary perfusion pressure (CPP), arterial blood gas results, right atrial pressure, and decompression aortic pressure.
Risk of bias assessment
The quality and risk of bias of included animal and human studies were assessed using the GRADE Assessment Tool [13]. The GRADE Assessment tool assesses quality of evidence in terms of study limitations, inconsistency, indirectness, imprecision and publication bias. These were graded with the consensus of 2 investigators (AFWH and YKT).
Statistical analysis
We presented the continuous variables as mean and standard deviation. Categorical variables were presented as frequencies and percentages. As there was substantial heterogeneity, we performed a random-effects meta-analysis on the odds ratio (OR) of survival to 30 days, comparing study arms randomized to PLR-CPR or C-CPR. Forest plots displayed individual and pooled ORs and 95% confidence intervals (95% CIs) for binary outcomes. Individual and pooled mean difference and 95% CI were presented for continuous outcomes. Two-tailed statistical significance was set at P<0.05. Between-study heterogeneity was assessed using the I2 statistic. Publication bias was assessed using funnel plots if there were 10 or more studies reporting the same outcome. All data analysis was conducted using the Cochrane Collaboration’s Review Manager (RevMan 5.4) Software Package.
RESULTS
Study selection and grading
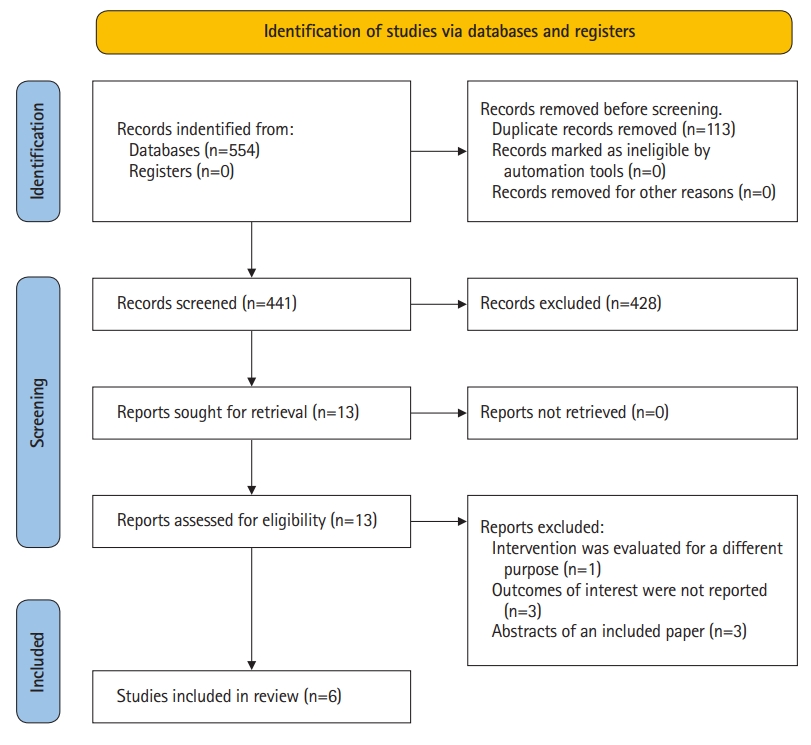
The search strategy yielded 554 studies, of which 113 studies were removed as duplicates. A further 428 studies were excluded after an initial screen of title and abstract as they did not report the use of PLR during CPR. Subsequently, 7 articles were excluded after full-text review. Finally, 6 eligible studies were included in our systematic review and meta-summary [7,8,14–17]. The study selection process and reasons for excluding the 7 excluded studies were illustrated in the PRISMA-P 2020 Flow Diagram (Fig. 1).
Characteristics of included studies
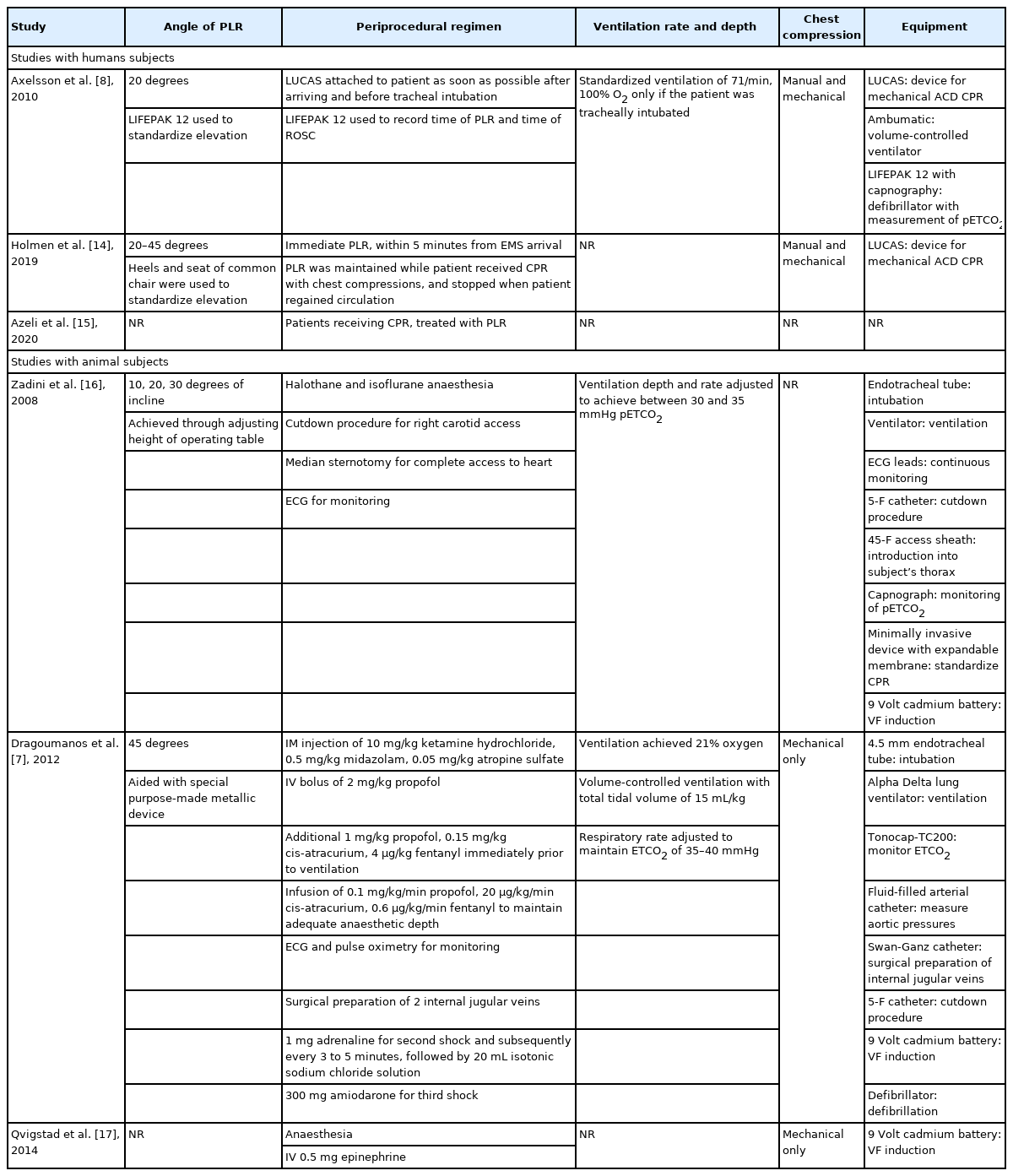
Of the 6 studies included in the review, Zadini et al. [16], Dragoumanos et al. [7] and Qvigstad et al. [17] were experimental trials involving animal subjects, Holmen et al. [14] was an observational study involving human patients, and the remaining 2 were experimental human trials. Among the experimental human trials, Azeli et al. [15] was a randomized controlled trial (RCT) and Axelsson et al. [8] was a secondary analysis of a cluster trial. A meta-summary of included studies was presented in Tables 1–4 [7,8,14–17].
Study design of human studies varied greatly. Axelsson et al. [8] was a secondary analysis of a cluster trial of mechanical CPR which aimed to assess partial pressure of end tidal carbon dioxide (pETCO2) levels that only included tracheal intubated patients with OHCA. The lone observational human study, Holmen et al. [14], retrospectively analysed OHCA cases over 3 years during which the emergency medical service (EMS) had implemented PLR within 5 minutes of CPR as part of their cardiac arrest protocol. Cases of non-compliance with the cardiac arrest protocol by the EMS study were then treated as the separate arm of the study. Meanwhile, Azeli et al. [15] randomized adult patients with OHCA to either PLR or C-CPR arms. However, details on the study arms and method of randomization were not reported.
For animal studies, Dragoumanos et al. [7] and Qvigstad et al. [17] were RCTs while the remaining study, Zadini et al. [16] was a single arm experimental trial. All animal studies involved porcine models of cardiac arrest where swines were subjected to a period of untreated ventricular fibrillation, which varied from 3 minutes to 8 minutes across study designs. However the aims across these 3 studies differed. Dragoumanos et al. [7] evaluated PLR’s effect on survival outcomes, hemodynamic performance and ROSC, while Qvigstad et al. [17] and Zadini et al. [16] solely evaluated hemodynamic performance and carotid blood flow respectively.
Experimental interventions and controls
The most common treatment among the 3 human studies was the bundling of PLR with an active compression-decompression device. Axelsson et al. [8] and Holmen et al. [14] implemented PLR as a 35 to 45 cm heel elevation which corresponded approximately to a 20- to 45-degree elevation at the hips. In contrast for the animal studies, Zadini et al. [16] implemented PLR as the Trendelenburg position where the pig was tilted 10, 20, and 30 degrees sequentially while Dragoumanos et al. [7] implemented PLR at 45 degrees (Table 2). All but one study used the supine position as the main control for comparison, as Zadini et al. [16] implementing a study design where each animal served as its own control. The remaining 5 studies had a proper control arm. However, amongst the 6 studies, Axelsson et al. [8], Holmen et al. [14] and Zadini et al. [16] and did not randomize their control arms, though it is worth noting that Holmen et al. [14] used propensity score matching to simulate balanced baseline prognostic factors.
Risk of bias
Quality of evidence was found to be high as evaluated by the GRADE framework in Table 5.
Study outcomes: primary and secondary outcomes
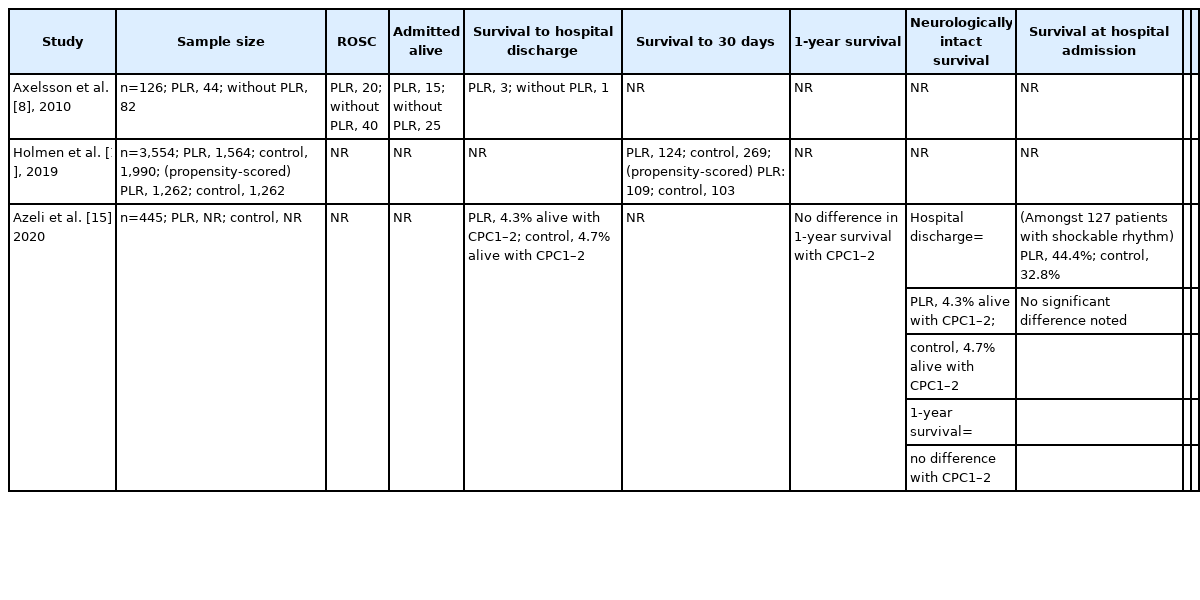
For the primary outcome of survival, 3 out of 6 studies included the outcomes of survival to 30 days/survival to discharge. These 3 studies all involved human patients. Meanwhile, only Axelsson et al. [8] and Azeli et al. [15] reported sufficient data for survival at hospital admission, and only Dragoumanos et al. [7] and Azeli et al. [15] reported on the outcome of neurologically intact survival. Dragoumanos et al. [7] was the sole study that accounted for the outcome of survival to 24 hours.
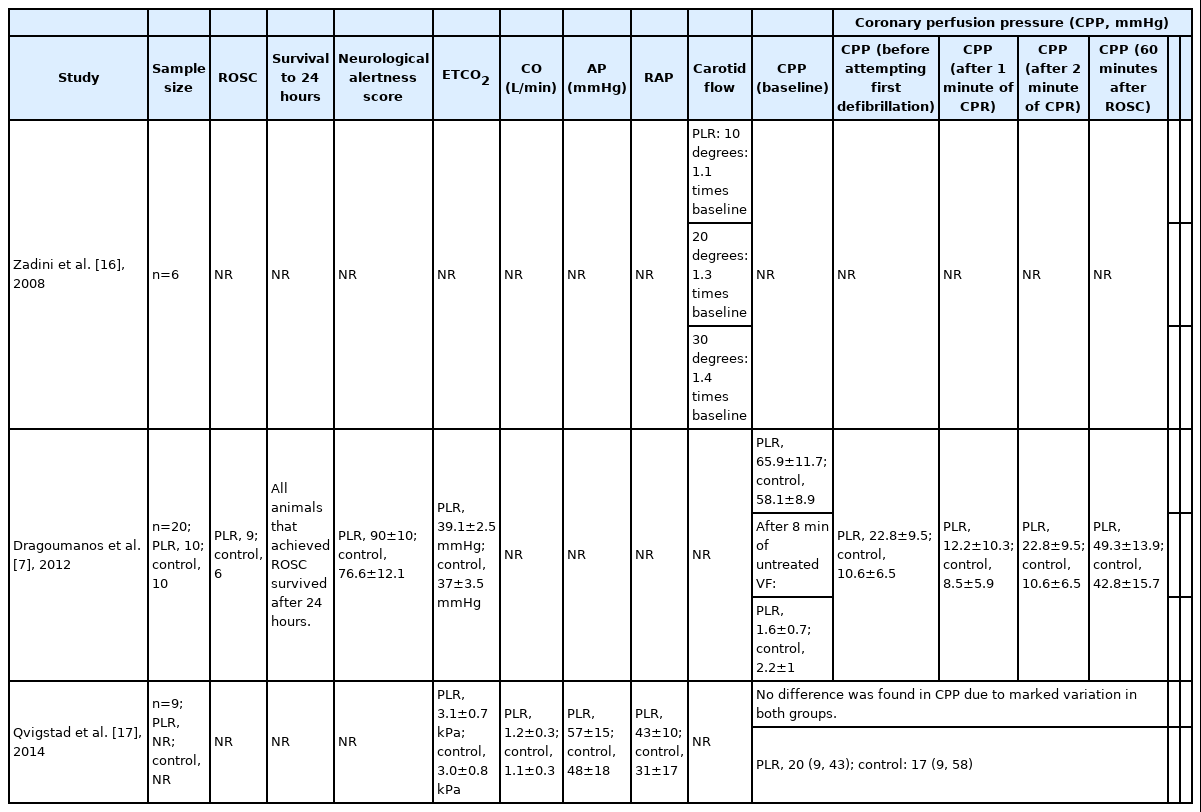
For the secondary outcomes, Axelsson et al. [8] and Dragoumanos et al. [7] detailed the outcome of ROSC. Similarly, Dragoumanos et al. [7] and Qvigstad et al. [17] reported on the outcome of CPP and Zadini et al. [16] was the sole study that investigated the impact of PLR on carotid blood flow.
Primary outcome: survival to hospital discharge/survival to 30 days
Azeli et al. [15] reported a lower proportion of PLR-CPR patients surviving, while both Axelsson et al. [8] and Holmen et al. [14] reported a higher proportion of patients that underwent PLR surviving at hospital discharge/to 30 days. However, all 3 comparisons were not statistically significant. It is also worth noting that Azeli et al. [15] reported survival to hospital discharge with cerebral performance category (CPC) 1 to 2 specifically.
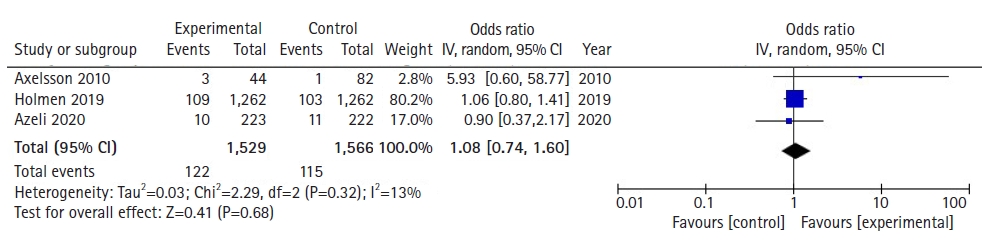
With regards to survival to 30 days or survival to hospital discharge, a total of 3,095 patients across 3 studies were assessed based on pooled survival outcomes. Meta-analytic estimates for survival showed no statistically significant benefit for patients where PLR-CPR was conducted in comparison to patients that underwent C-CPR, as shown in Fig. 2 (OR, 1.08; 95% CI, 0.74−1.60; P=0.68). There was low heterogeneity (I2=13%).
Primary outcome: survival at hospital admission
Only Axelsson et al. [8] and Azeli et al. [15] reported on the outcome of survival at hospital admission. Both studies reported a higher proportion of patients that underwent PLR surviving, though not reaching statistical significance. Both studies concluded that PLR was not shown to have a significant effect on survival outcomes.
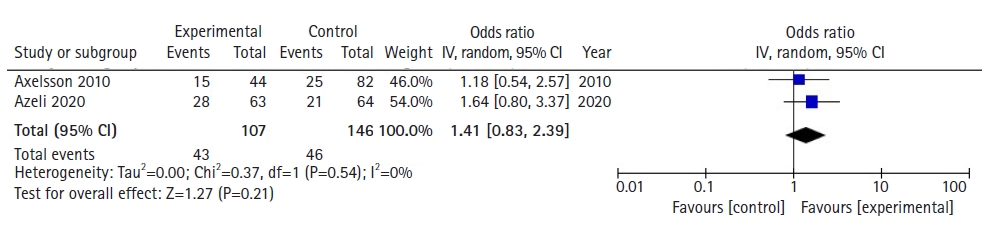
With regards to survival at hospital admission, a total of 253 patients across 2 studies were assessed based on pooled survival outcomes. Meta-analytic estimates for survival showed no statistically significant benefit for patients where PLR-CPR was conducted in comparison to patients that underwent C-CPR, as shown in Fig. 3 (OR, 1.41; 95% CI, 0.83−2.39; P=0.21). There was low heterogeneity (I2=0%).
Primary outcomes: survival to 24 hours and neurologically intact survival
For the outcome of survival to 24 hours, only Dragoumanos et al. [7] reported survival to 24 hours. It revealed that all animals that achieved ROSC survived after 24 hours, though not to statistical significance (P=0.121). Meanwhile, only Azeli et al. [15] and Dragoumanos et al. [7] reported neurologically intact survival. No statistical significant difference was found between PLR-CPR and C-CPR groups in Azeli et al. [15] (P=0.81). However, in Dragoumanos et al. [7], animals with PLR-CPR (90±10) were shown to have a significantly higher neurological alertness score compared to those with C-CPR (76.6±12.1, P=0.037).
Secondary outcome: ROSC
Axelsson et al. [8] and Dragoumanos et al. [7] were the only studies that reported ROSC, with Dragoumanos et al. [7]’s results favouring PLR while Axelsson et al. [8]’s results favoured C-CPR. However, both results were not statistically significant.
Secondary outcome: CPP
Dragoumanos et al. [7] and Qvigstad et al. [17] were the only studies to report CPP. Both studies found that CPP in the PLR group was higher compared to that of the C-CPR group. However, this difference was only statistically significant in Dragoumanos et al. [7].
Secondary outcome: carotid blood flow
Zadini et al. [16] was the only study to report carotid blood flow. It concluded that an increase of up to 1.4 times in carotid blood flow can be attained during CPR in the Trendelenburg position, although only 20 and 30 degrees of Trendelenburg showed a statistically significant increase from the 0 degrees of tilt in pigs.
Publication bias
Publication bias could not be assessed as there were fewer than 10 studies reporting each outcome.
DISCUSSION
In recent years, PLR-CPR has garnered significant interest in its role during CPR for the treatment of SCA. While there have been studies focusing on its predictive value for fluid responsiveness and mortality benefit on septic shock patients, there has been no systematic review and meta-analysis on the survival benefits of PLR-CPR in SCA patients to date. In this study, we describe the survival outcomes of SCA subjects with PLR-CPR, and report several important findings that might have potential implications in clarifying the effectiveness of PLR-CPR in SCA patients.
First, meta-analytic estimates showed no statistically significant difference for the outcomes of survival to 30 days or survival to hospital discharge as well as survival at hospital admission when comparing PLR-CPR to C-CPR. Second, there was no significant difference between PLR-CPR and C-CPR groups for the outcome of ROSC in both Axelsson et al. [8] and Dragoumanos et al. [7]. Third, CPP was reported to be higher in the PLR arm compared to the C-CPR arm in both Dragoumanos et al. [7] and Qvigstad et al. [17]. However, the difference was only statistically significant in Dragoumanos et al. [7]. Fourth, Zadini et al. [16] reported a statistically significant increase of carotid blood flow during CPR in the Trendelenburg position.
Our pooled analysis suggests that there was no significant difference for the outcomes of survival to 30 days or survival to hospital discharge as well as survival to hospital admission when comparing PLR-CPR to C-CPR. This is consistent with existing literature, where 3 human studies, Holmen et al. [14], Azeli et al. [15] and Axelsson et al. [8], found that the difference between PLR-CPR and C-CPR groups was statistically insignificant (P=0.69, P=0.81, P=0.12, respectively). Moreover, Azeli et al. [15] and Axelsson et al. [8] also reported no significant difference between the PLR-CPR and C-CPR groups for the outcome of survival to hospital admission (P=0.18, P=0.69). A similar trend was noted for neurologically intact survival, where Azeli et al. [15] reported no difference in one year survival of patients with CPC scores 1 to 2 for both PLR-CPR and C-CPR groups (P=0.81). This may be due to PLR delaying other important interventions, such as defibrillation [14]. Further, studies have shown that the blood volume mobilized by leg-raising is unpredictable, and is especially unreliable in severely hypovolemic patients [18,19]. This is supported by Axelsson et al. [8] and Dragoumanos et al. [7], which both reported no significant difference between PLR-CPR and C-CPR groups for ROSC (P=0.85, 0.121, respectively). These findings allude to the unpredictability of the augmentation of artificial circulation by PLR during external cardiac compression. Importantly, it emphasises a need for more trials to be conducted to investigate such survival outcomes [10,14].
However, there are studies that indicate a trend favouring PLR-CPR to C-CPR for certain situations. An animal study, Dragoumanos et al. [7], found that all animals that achieved ROSC survived within 24 hours, though not to the point of statistical significance. In the same study, animals with PLR-CPR were also shown to have a significantly higher neurological alertness score compared to those with C-CPR (P=0.037). These findings might suggest PLR-CPR acts as a rapid intravenous volume expander to shift blood from the lower extremities towards the intrathoracic compartment [14]. According to Reuter et al. [20], a 45-degree leg elevation for 4 minutes increases right and left ventricular preload and, by definition, the stroke volume, if the heart is preload dependent. Indeed, 2 animal studies, Dragoumanos et al. [7] and Qvigstad et al. [17], demonstrated this particular effect through a rise in ETCO2 values for their PLR arms. As ETCO2 values are proven quantitative predictors of stroke volume [21], a rise in ETCO2 values for PLR-CPR suggests increased blood flow during the PLR technique for CPR [19,22–24]. Additionally, Dragoumanos et al. [7] reported a statistically significant increase in CPP for the PLR-CPR group and Zadini et al. [16] recorded a statistically significant increase (of up to 1.4 times) of carotid blood flow in the Trendelenburg position during CPR. Given the positive outcomes for PLR in animal studies, a case can be made to trial PLR-CPR in the real world.
The contrast in survival and perfusion outcomes between animal and human studies underscores the conflicting evidence behind the effectiveness of PLR-CPR and spotlights the ongoing debate on how PLR may improve outcomes of resuscitation maneuvers in CPR [8]. While there exist studies that suggest the purported benefits of PLR-CPR [7,16,17], such conclusions need to be made with caution. This is due to 2 reasons. First, the PLR-favored outcome of survival to 24 hours in Dragoumanos et al. [7] is statistically insignificant. Second, studies which showed relatively favourable survival outcomes for the PLR group were animal studies [7,16,17]. There is an inherent difficulty in extrapolating outcomes from animal studies to humans due to fundamental differences in anatomy and physiology [25].
While there is conflicting evidence on the effectiveness of PLR as a treatment, there is general consensus that PLR is a reliable predictor of fluid responsiveness among patients with circulatory failure [26]. An observational study by Preau et al. [27] notes that certain indices induced by PLR are accurate for predicting fluid responsiveness in nonintubated patients with severe sepsis or acute pancreatitis. Further, PLR has been recommended as part of haemodynamic monitoring in recent international recommendations [28,29]. In its capacity as a predictor for fluid responsiveness, PLR is a useful technique to be performed.
PLR appears promising as a fast and accessible technique, with generally good outcomes in animal studies. However, in human studies, we found no statistically significant difference between C-CPR and PLR-CPR. In the current literature, there exists a paucity of data as shown by the fact that our study was only able to pool 2 studies for meta-analysis. Moving forward, PLR-CPR should continue to be used as a predictor for fluid responsiveness in patients with circulatory failure. However, medical professionals should exercise caution if the aim of performing PLR-CPR is to improve survival outcomes in SCA patients. We recommend more studies to be conducted focusing on survival outcomes in order to best elucidate the effect of PLR-CPR.
The findings of this analysis should be interpreted in the context of known limitations. First, our findings are limited by the paucity of studies and data, with only 6 included studies in our systematic review. With regards to survival outcomes, not all studies reported survival outcomes, and the 2 human studies that accounted for survival outcomes had contrasting results. Second, survival outcomes might be influenced by many confounding factors, which might unduly influence results from the studies. Third, PLR-CPR may result in false negative findings in patients with raised intra-abdominal pressure, which our included studies did not take into account. Finally, significant heterogeneity is expected due to the paucity of data and varying methodologies utilized in the included studies. For example, Zadini et al. [16] implemented PLR-CPR in a Trendelenburg position in contrast to the standardized horizontal plane used in other studies. Additionally, the inclusion criteria of both animal and human studies in the analysis contributed to inherent heterogeneity in our results. These factors limited our ability to generalize the effect of PLR.
In conclusion, PLR is an experimental technique used in CPR that has purported benefits for SCA patients due to its ease of applicability and plausible physiological basis for improving cardiac output during CPR. Despite several animal studies showing benefit from PLR-CPR, there is no human data supporting its use in human cardiac arrest. Future research needs to ascertain the best positioning during CPR to optimize clinical outcomes.
Notes
FUNDING
AFWH was supported by the Estate of Tan Sri Khoo Teck Puat (Khoo Clinical Scholars Programme), Khoo Pilot Award (KP/2019/0034), Duke-NUS Medical School and National Medical Research Council (NMRC/CS_Seedfd/012/2018). CHS was supported by the National University of Singapore Yong Loo Lin School of Medicine’s Junior Academic Faculty Scheme.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHORS’ CONTRIBUTIONS
Conceptualization: FZ, MEHO, AFWH; Data curation: YKT, YM; Methodology: YKT, BYQT, CHS, MEHO, AFWH, YM; Project administration: YKT; Resources: all authors; Writing–original draft: YKT, BYQT, CHS, AFWH, YM; Writing–review & editing: all authors.